Atoms To Mass In Grams Converter Chart
 Apr 21, 2012 The entire suite taken from the Czech Republic Orchestra recorded 6-6-1998 00:00 Princess Mononoke, symphonic suite: Legend of Ashitaka 05:50 Princess Monono. Jun 26, 2008 Mix - Joe Hisaishi / Princess Mononoke Symphonic Suite YouTube 魔女の宅急便 オーケストラ in武道館 Joe Hisaishi in Budokan - Duration: 9:42. Localhorst6822 10,826,004 views.
Apr 21, 2012 The entire suite taken from the Czech Republic Orchestra recorded 6-6-1998 00:00 Princess Mononoke, symphonic suite: Legend of Ashitaka 05:50 Princess Monono. Jun 26, 2008 Mix - Joe Hisaishi / Princess Mononoke Symphonic Suite YouTube 魔女の宅急便 オーケストラ in武道館 Joe Hisaishi in Budokan - Duration: 9:42. Localhorst6822 10,826,004 views.
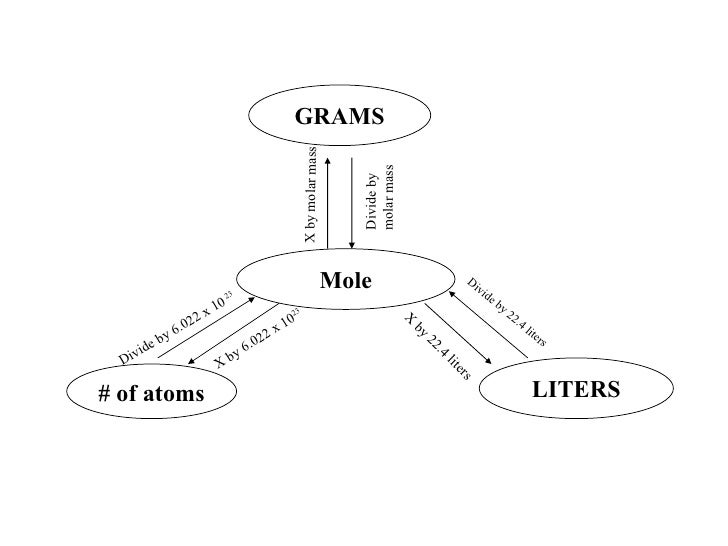
Avogadro's number is one of the most important constants used in chemistry. It is the number of particles in a single mole of a material, based on the number of atoms in exactly 12 grams of the isotope carbon-12. Although this number is a constant, it's experimentally determined, so we use an approximate value of 6.022 x 1023. So, you know how many atoms are in a mole. Here's how to use the information to determine the mass of a single atom.
- Calculate Mass In Grams
- Atoms To Mass In Grams Converter Chart To Inches
- Atoms To Grams Conversion Formula
- Then you can convert atoms to mass in grams or kilograms. The table below gives mass of some of the atoms. How would you convert Atoms to. ›› Want other units? Enter two units to convert From: To: ›› Definition: Atom This site uses an exact value of 6.0221415 x 10 23 for Avogadro's number. This is the number of atoms in 1 mole of a.
- Instant free online tool for Atomic mass unit to gram conversion or vice versa. The Atomic mass unit u to gram g conversion table and conversion steps are also listed. Also, explore tools to convert Atomic mass unit or gram to other weight and mass units or learn more about weight and mass conversions.
Avogadro's Number Example Problem: Mass of a Single Atom
Instantly Convert Newtons (N) to Grams (g) and Many More Mass Conversions Online. Newtons Conversion Charts. Many Other Conversions.
Question: Calculate the mass in grams of a single carbon (C) atom.
Solution
To calculate the mass of a single atom, first look up the atomic mass of carbon from the periodic table.
This number, 12.01, is the mass in grams of one mole of carbon. One mole of carbon is 6.022 x 1023 atoms of carbon (Avogadro's number). This relation is then used to 'convert' a carbon atom to grams by the ratio:
mass of 1 atom / 1 atom = mass of a mole of atoms / 6.022 x 1023 atoms
Calculate Mass In Grams
Plug in the atomic mass of carbon to solve for the mass of 1 atom:
mass of 1 atom = mass of a mole of atoms / 6.022 x 1023
mass of 1 C atom = 12.01 g / 6.022 x 1023 C atoms
mass of 1 C atom = 1.994 x 10-23 g
Answer
The mass of a single carbon atom is 1.994 x 10-23 g.
Atoms To Mass In Grams Converter Chart To Inches
Applying the Formula to Solve for Other Atoms and Molecules
Although the problem was worked using carbon (the element upon which Avogadro's number is based), you can use the same method to solve for the mass of an atom or molecule. If you're finding the mass of an atom of a different element, just use that element's atomic mass.
If you want to use the relation to solve for the mass of a single molecule, there's an extra step. You need to add up the masses of all of the atoms in that one molecule and use them instead.
Let's say, for example, you want to know the mass of a single atom of water. From the formula (H2O), you know there are two hydrogen atoms and one oxygen atom. You use the periodic table to look up the mass of each atom (H is 1.01 and O is 16.00). Forming a water molecule gives you a mass of:
1.01 + 1.01 + 16.00 = 18.02 grams per mole of water
and you solve with:
mass of 1 molecule = mass of one mole of molecules / 6.022 x 1023
mass of 1 water molecule = 18.02 grams per mole / 6.022 x 1023 molecules per mole
mass of 1 water molecule = 2.992 x 10-23 grams
Atoms To Grams Conversion Formula
Converting atoms to grams is an essential process in basic chemistry and forms the foundation for the more difficult calculations used in more advanced chemistry. The conversion requires a fundamental understanding of Avogadro’s Number, atomic weights, dimensional analysis and the definition of a mole of a substance. Using these items, if you know how many atoms of a substance you are dealing with, you can easily convert this to grams using the following process.
Instructions
Once you have done this a few times, you will see that multiplying the number of atoms and dividing the result by Avogadro’s number (6.02 x 10^23) will suffice.
Use only as many significant figures as the least precise number in your calculation. In this example, 14 is two significant figures, so our answer is to two figures as well.
For the purpose of this demonstration we will assume that we are working with 14 atoms of carbon. Write “14 atoms C†on the top left of your scratch paper.
Avogadro’s number (6.02 x 10^23) is the number of particles of a substance in one mole (mol) of that substance. There are 6.02 x 10^23 atoms of carbon in a mole of carbon and 6.02 x 10^23 molecules of water in a mole of water. Because you are using dimensional analysis to cancel out “atoms,†to the right of what you wrote in Step 1 write as a fraction, “1 mol C / 6.02 x 10^23 atoms†and prepare to multiply across, such that your equation looks like this so far:
14 atoms C 1 mol x ------------------------------- 6.02 x 10^23 atoms C
Refer to the periodic table of elements and find the atomic weight for the substance you are working with, rounding to the appropriate number of significant digits. In this case, carbon has an atomic weight of 12.0 atomic mass units (amu). The molar mass (in grams) of any substance is always numerically equal to its formula weight (in amu), so for carbon, there are 12.0 grams (g) in one mole of carbon. Write this as a fraction to the right of Step 2, again multiplying. Also, put an equal sign to the far right. It should look like this:
14 atoms C 1 mol 12.0 g C x ------------------------------ x -------------- = 6.02 x 10^23 atoms C 1 mol C
Because the units in fractions get treated the same as numbers do, the “atoms C†from Steps 1 and 2 cancel each other out and the “mol C†from Steps 2 and 3 cancel out, leaving you with grams (g) as the unit of measurement your answer will be in. This is a good way to check your work.
Multiply across the top to get 168 g C and across the bottom to get 6.02 x 10^23.
Divide the top by the bottom, minding significant figures, to get 2.8 x 10^22 grams of carbon in 14 atoms of carbon.
- “Chemistry: The Central Science;†Brown, Bursten, & LeMay; 1997